
Unveiled at CPHI 2024, Morpho represents the new brand identity of Alfasigma’s CDMO (Contract Development and Manufacturing Organization). Inspired by the ancient Greek word to shape and transformation, Morpho embodies our commitment to excellence transforming row materials into high quality pharmaceutical products.
With over 60 years of expertise and a strong international network across Europe and the USA, Morpho adopts a customer-centric approach, managing third-party production with the same care and high standards applied to Alfasigma’s own branded products.



Morpho intends to be recognized as leading Tech Driven Specialty CDMO belonging to a global pharmaceutical company, focused on value-added development and manufacturing technologies. Through its three divisions PharmaTech, Fine Chemicals (Biosint), and Dietary Supplements & Medical Food (US), Morpho provides end-to-end pharmaceutical and nutraceutical solutions, tailored to meet the specific needs of its partners worldwide.
MORPHO IN A NUTSHELL
Morpho’s capabilities span a wide range of pharmaceutical forms—including sterile injectables, vaccines, oral solids, semi-solids, and more—supported by advanced formulation research, clinical batch preparation, and process validation services.
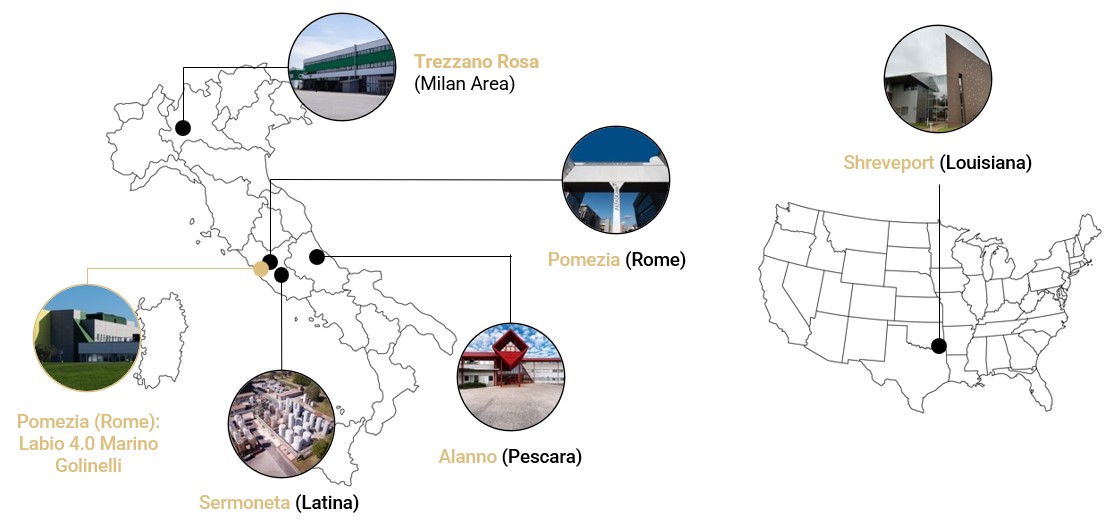
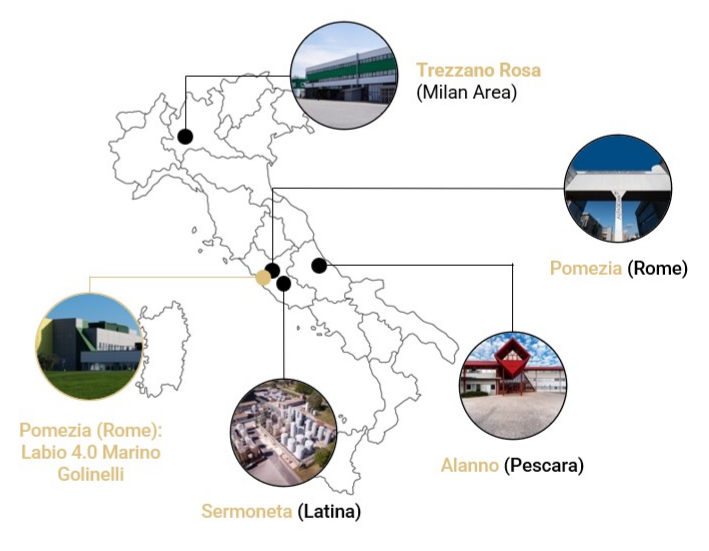
Morpho’s development and manufacturing network offers specialized capabilities to meet a wide range of pharmaceutical, medical foods, and dietary supplements needs, with strategic locations across Europe and the USA.

Operations is a tablet and hard capsule manufacturing factory with bottle, blister, and stick pack packaging capabilities
Quality is a key priority in every aspect of our business. Our patients rely on us to provide and deliver the highest quality standards, and we are committed to living up to those expectations.


Supply Chain capabilities allow our team to procure and maintain in house inventory of raw materials, packaging components, and finished goods.
Alfasigma USA, Inc.
Shreveport Factory
2008 Claiborne Ave
Shreveport, LA 71103
U.S.A.
Phone
+1 (318) 425-9606
Sylvia Ortega Martinez, Ph.D
Head of Morpho - US
Phone
+1 (224) 300-0904